Abstract
INTRODUCTION: Patients (pts) with polycythemia vera (PV) have a significantly higher risk of thrombosis than the general population, and cardiovascular (CV) events are the main cause of death. Pts with PV also report reduced quality of life (QoL) due to high symptom and psychological burden. This analysis of the LANDMARK 2.0 survey examines patterns related to assessments of CV risk and PV-related psychological burden in pts with PV.
METHODS: LANDMARK 2.0 is an online survey of MPN pts and treating physicians that was conducted in 11 countries between April 2021-May 2022. This analysis included pts with PV aged 18-89 years (yrs), and physicians who were actively managing PV pts within 12 months (mos) prior to the survey. Pts were stratified into 3 therapy (tx) stages: currently receiving first-line cytoreductive tx for ≤1 yr (PV-C), currently receiving second-line tx for ≤1 yr (PV-I), or currently receiving any line of tx for >1 yr (PV-M).
RESULTS: Overall, 133 physicians and 274 pts with PV (PV-C, n=86; PV-I, n=26; PV-M, n=162) completed the survey. At survey completion, the median age of pts was 63, 59, and 64 yrs in the PV-C, PV-I, and PV-M groups, respectively, and the median time since PV diagnosis was 10.8, 39, and 91 mos, respectively. Most common treatments across all tx stages were hydroxyurea and ruxolitinib. Phlebotomies were received by 53%, 31%, and 29% pts in PV-C, PV-I, and PV-M stages, respectively, with a mean number of 4.2, 3.4, and 4.6 phlebotomies, respectively, in the past 12 mos.
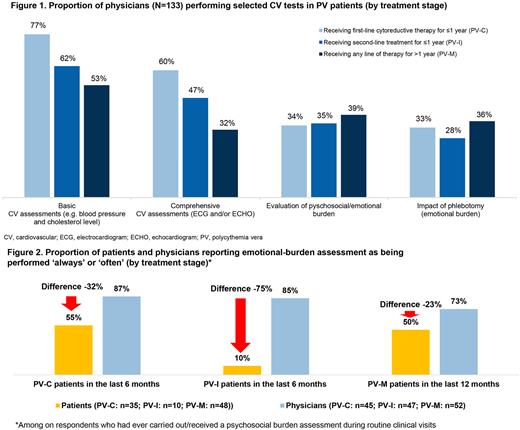
During routine visits, basic CV assessment (e.g. blood pressure and cholesterol) was ever performed by 53% of physicians treating PV-M pts, 77% physicians treating PV-C pts, and 62% physicians treating PV-I pts, and comprehensive CV assessment (i.e. electrocardiogram and/or echocardiogram) was ever performed by 32%, 60%, and 47% of physicians, respectively (Fig 1). Among physicians who never conducted basic (n=21) or comprehensive (n=40) CV assessments, 38% and 43%, respectively, considered the assessment unnecessary, 38% and 15%, respectively, cited insufficient time as reason, and 36% and 19%, respectively, cited insufficient resources. Among all physicians, 68% and 63%, respectively, did not think that basic or comprehensive CV tests could be used more effectively in pt management. In contrast to physicians' responses, only 55% of pts across tx stages reported having ever received basic CV tests during routine visits. Fewer pts in PV-M received advice on reducing CV risk (45% in the last 12 mos) vs pts in PV-C and PV-I (73% and 69%, respectively, in the last 6 mos).
When asked about the burden of PV on QoL on a scale of 1 (no impact) to 5 (significant impact), a large proportion of pts reported an impact of 3-5 on emotional well-being and mental health, in particular those in PV-C (66%) and PV-I (80%). Overall, 38% of pts rated the impact of phlebotomy on QoL as 3-5, but only around a third of physicians (PV-C, 33%; PV-I, 28%; PV-M, 36%) reported ever having assessed the emotional burden of phlebotomy.
Among respondents who had ever carried out/received a psychosocial burden assessment during routine visits, there was a discordance in the perceived frequency of these tests, with fewer pts than physicians reporting the assessment being performed 'always' or 'often' (discordance of -32%, -75% and -23% in the PV-C, PV-I, and PV-M groups, respectively; Fig 2). With respect to the support received for PV-related psychological burden, only 52% of pts reported being 'somewhat' or 'very' satisfied. More pts vs physicians reported a lack of communication on self-help strategies in PV-I (36% vs 12%) or on the rationale for having performed specific tests in PV-I (20% vs 8%) and PV-C (24% vs 9%).
CONCLUSIONS: Whilst international recommendations recognize the need to control CV risk factors, there is no standard recommendation on how to monitor CV risk in PV, highlighting an unmet need. These results show that CV-risk testing and education on CV-risk minimization are not routinely performed throughout the treatment continuum, in particular in pts who are stabilized on long-term maintenance tx. Additionally, our findings indicate a discordance between pts and physicians in the perceived importance of PV-related QoL assessments. Pt care may be enhanced with regular assessments throughout treatment and increased communication between pts and physicians.
Study funding: Novartis
Disclosures
Kiladjian:Incyte: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; AOP Orphan: Membership on an entity's Board of Directors or advisory committees. Ross:Keros: Consultancy; Celgene: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria. Foltz:Amgen: Current Employment, Divested equity in a private or publicly-traded company in the past 24 months; Novartis: Honoraria; BMS: Honoraria, Research Funding; Sierra Oncology: Other: Medical Writing, Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Constellation: Research Funding; CTI Biopharma: Research Funding; Paladin Labs Inc: Membership on an entity's Board of Directors or advisory committees, Other: Committee; Sierra: Research Funding. Busque:Novartis: Consultancy. Heidel:Celgene/BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; CTI: Consultancy, Research Funding; AOP: Consultancy. Koehler:Novartis: Consultancy. Palumbo:Abbvie: Honoraria; Novartis: Consultancy, Honoraria; BMS/Celgene: Consultancy; AOP: Consultancy; AstraZeneca: Consultancy. Breccia:Novartis, Incyte, Pfizer, BMS, Abbvie: Honoraria. Komatsu:Novartis, Takeda: Speakers Bureau; Abbvie Inc, Celgene, Japan Tabacco Inc: Honoraria; FUJIFILM Wako, Chemicals, Meiji Seija Pharma, Perseus Proteomics Inc., Chugai Pharmaecutical, Kyowa-Hakko Kirin, Sumitomo Dainippon, Pharma: Research Funding; Otsuka Pharmaceutical, Takeda Pharmaceutical: Research Funding; PharmaEssentia Japan K.K.: Membership on an entity's Board of Directors or advisory committees. Kirito:Novartis: Honoraria. Rovo:AstraZeneca: Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria; Alexion: Consultancy, Research Funding; CSL Behring: Research Funding; Novartis: Consultancy, Research Funding; OrPhaSwiss GmbH: Membership on an entity's Board of Directors or advisory committees; Swedish Orphan Biovitrum AG: Membership on an entity's Board of Directors or advisory committees. Petruk:Abbvie, Novartis, Pharmessentia BMS: Consultancy. Zuurman:Novartis Pharma AG: Current Employment. Harrison:EHA: Other: Leadership role; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Geron: Membership on an entity's Board of Directors or advisory committees; Promedior: Membership on an entity's Board of Directors or advisory committees; Keros: Consultancy; Galecto: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; MPN voice: Other: Leadership role; AOP Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sierra: Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings, Research Funding; Galacteo: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal